Light is an electromagnetic radiation.
According to Planck’s theory, when energy is supplied to the electrons of an atom, which are orbiting around the nucleus, the electrons move from their ground state to a higher energy level. Since this new level is not stable for these electrons, they tend to return to their ground state once the energy source is removed. According to Planck’s theory, the energy stored during this transition process is released in the form of light, as packets of energy called photons, when the electrons return from the higher level to the lower one.
The wavelength of the emitted light is directly proportional to the amount of energy released. This relationship is expressed with the help of a constant called Planck’s constant, and it is shown in the following equation:
E=hν
In this equation, h is Planck’s constant, ν is the frequency (which is inversely related to the wavelength) of the emitted light, and E is the energy.
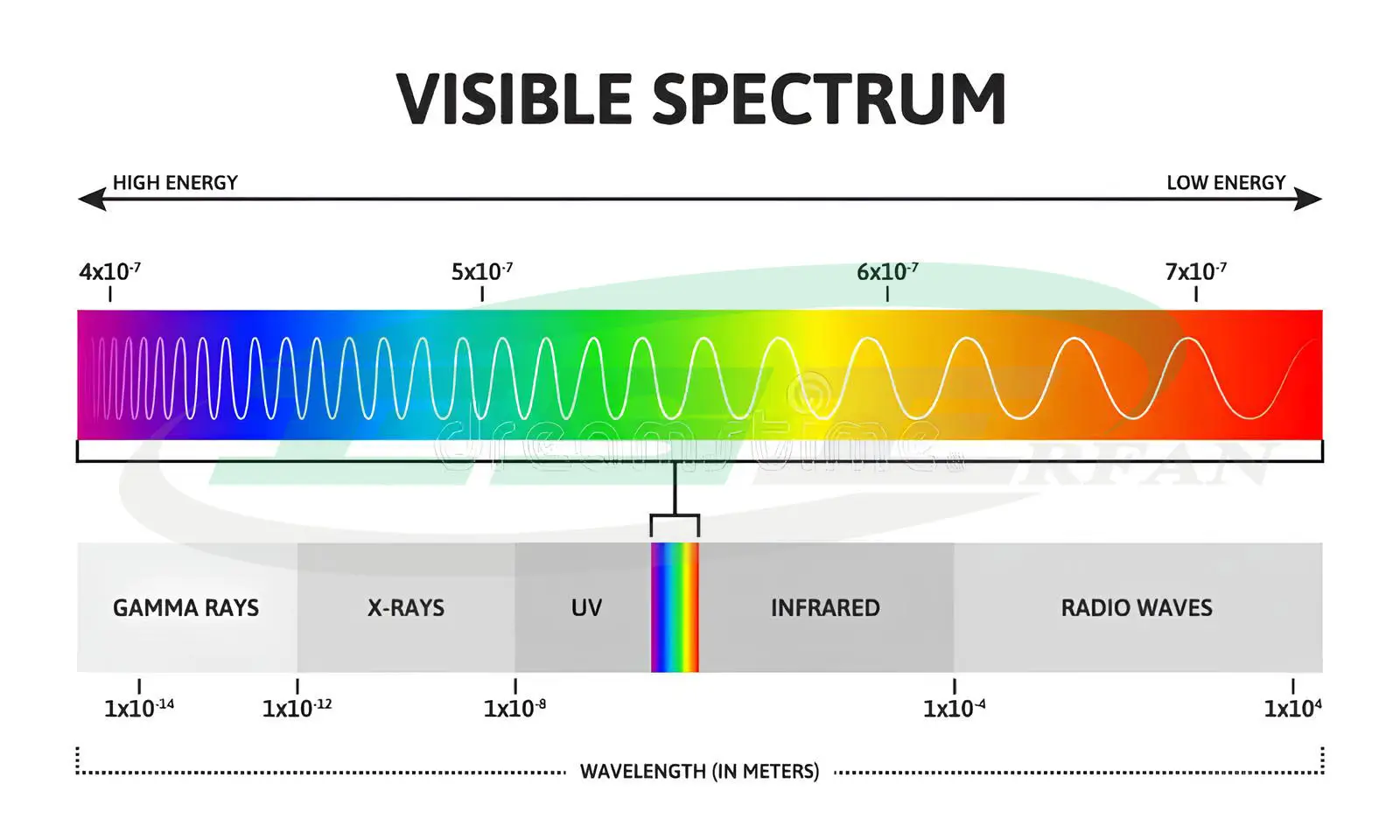
Light Emission as Electromagnetic Waves
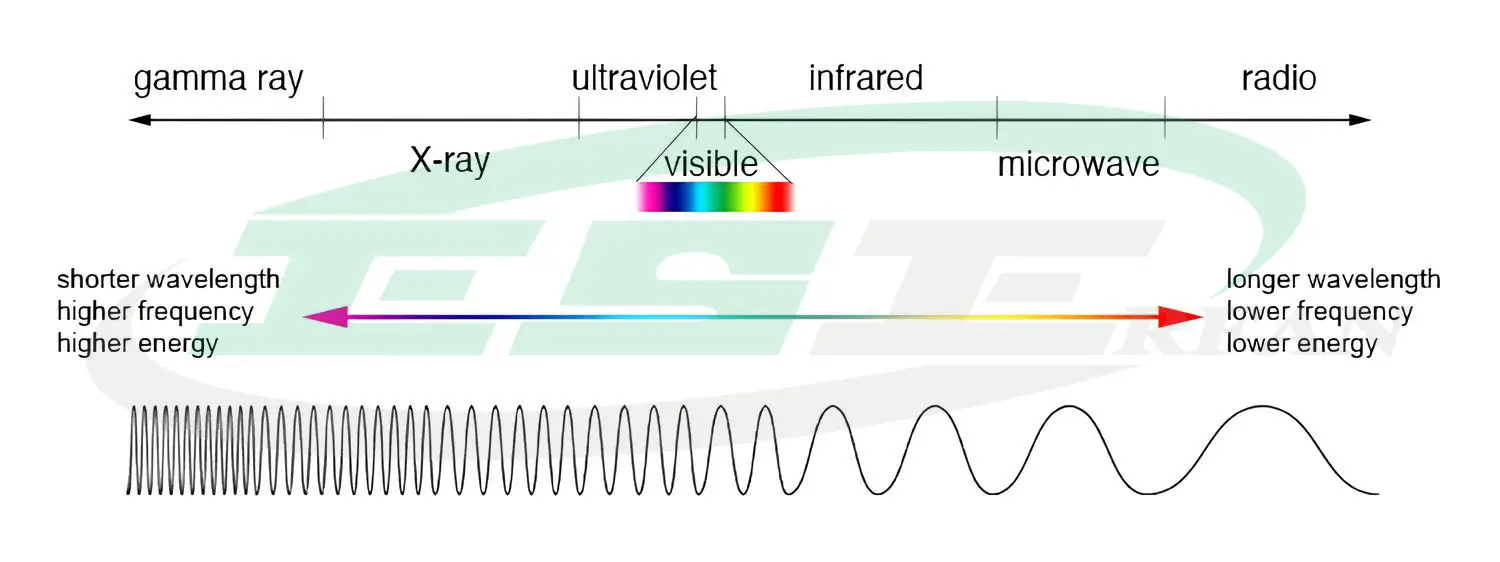
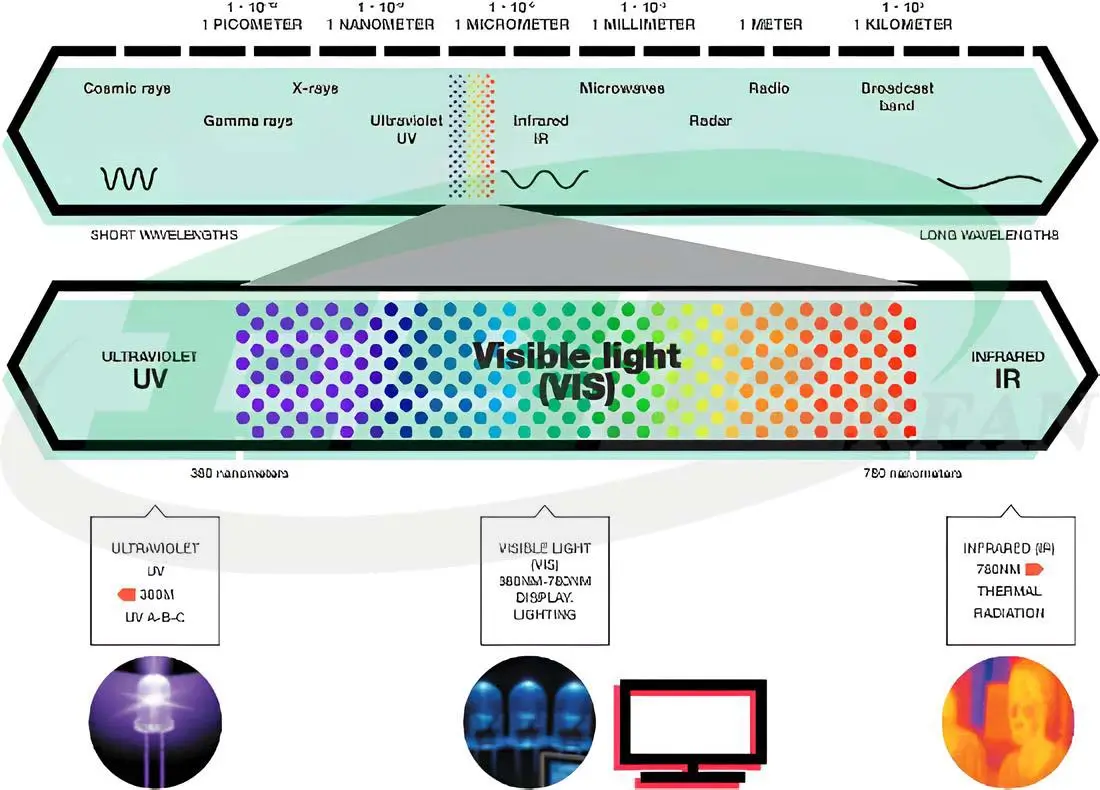
In the theory of light emission as electromagnetic waves, light is a form of electromagnetic energy. Electromagnetic waves with wavelengths between 780-380 nanometers, which are visible to the human eye, are referred to as visible light. This light is a combination of different wavelengths.
At both ends of the visible light spectrum, there are infrared waves and ultraviolet rays.
- Infrared Waves:
These waves fall within the range of 1000-780 nanometers and are not visible to the human eye. When infrared waves hit an object, they are absorbed and converted into heat. These waves are the primary means by which solar heat is transferred to the Earth. -
Ultraviolet Rays (UV):
Ultraviolet rays, commonly referred to as UV, are essential for many vital activities on Earth. They fall within the range of 380-100 nanometers and are divided into three types:- UV-A
- UV-B
- UV-C
These waves have various effects on the human body and the environment. For example, UV-A rays penetrate the skin more deeply and can cause premature aging and skin cancer, while UV-B rays can damage DNA and lead to severe sunburns. UV-C rays, which are largely absorbed by the ozone layer, are the most dangerous type of UV radiation.
What is Light?
The UV-A spectrum is responsible for tanning and falls within the range of 380–315 nanometers.
The UV-B spectrum causes skin redness, inflammation, and sunburn and is in the range of 315–280 nanometers.
The UV-C spectrum, within the range of 280–100 nanometers, destroys cellular structures and is therefore used in sterilization and disinfection with ultraviolet radiation.
Despite the positive effects of ultraviolet radiation (for instance, the UV-B spectrum helps produce vitamin D in the body), excessive exposure to this radiation can be harmful to the body.
The ozone layer absorbs a significant amount of the sun’s ultraviolet radiation, especially the UV-C spectrum.